Reduced synaptic vesicle protein degradation at lysosomes curbs tbc1d24/sky-caused neurodegeneration
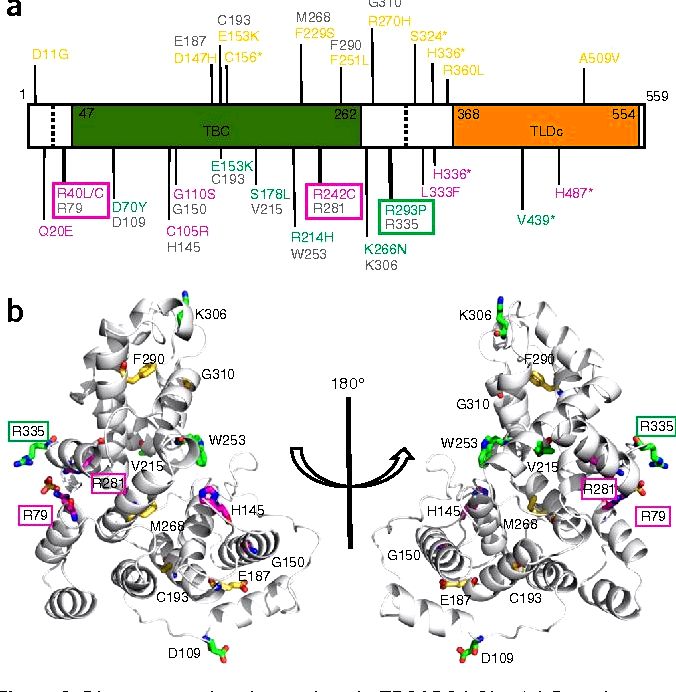
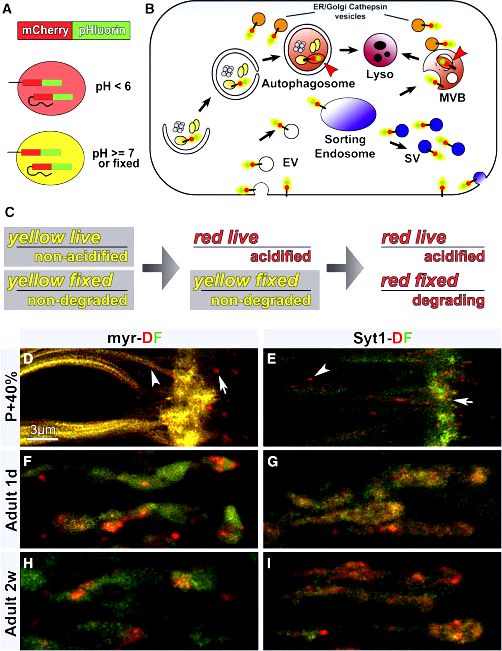
Synaptic demise and accumulation of structural proteins are regarded as common features in neurodegeneration. However, the mechanisms through which synaptic proteins start remain elusive. Within this paper, we study Drosophila melanogaster missing active TBC1D24/Skywalker (Sky), a protein that in humans causes severe neurodegeneration, epilepsy, and DOOR (deafness, onychdystrophy, osteodystrophy, and mental retardation) syndrome, and identify endosome-to-lysosome trafficking like a mechanism for degradation of synaptic vesicle-connected proteins. In fly sky mutants, synaptic vesicles traveled excessively to endosomes. Using chimeric fluorescent timers, we reveal that synaptic vesicle-connected proteins were more youthful typically, suggesting that older proteins tend to be more efficiently degraded. Utilizing a genetic screen, we discover that reducing endosomal-to-lysosomal trafficking, controlled through the homotypic fusion and vacuole protein sorting (HOPS) complex, saved the neurotransmission and neurodegeneration defects in sky mutants. Consistently, synaptic vesicle proteins were older in HOPS complex mutants, which mutants also demonstrated reduced neurotransmission. Our findings define a mechanism by which synaptic transmission is facilitated by efficient protein turnover at lysosomes and identify a possible technique to suppress defects as a result of TBC1D24 mutations in humans.
Resourse: http://jcb.rupress.org/content/207/4/