Synaptic transmission and plasticity require ampa receptor anchoring via its n-terminal domain
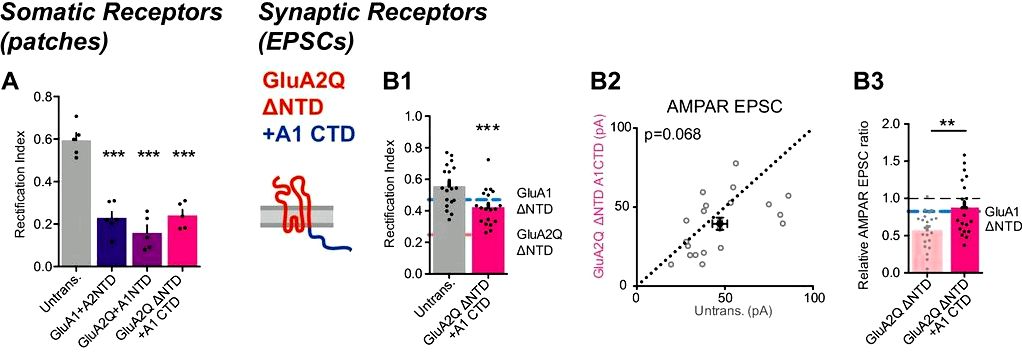
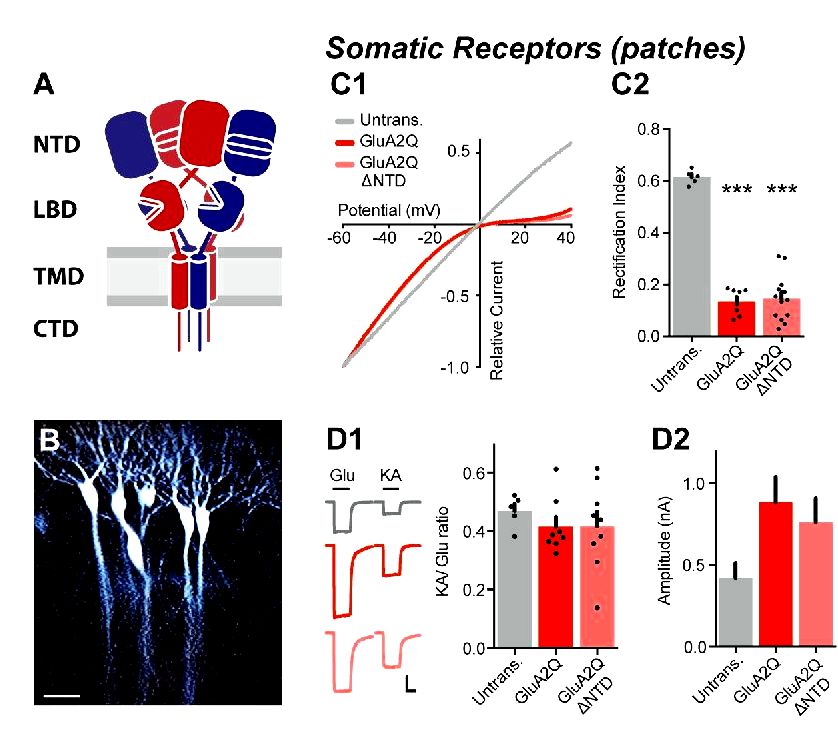
3) We’ve investigated NTD-dependent AMPAR anchoring utilizing a save strategy in AMPAR conditional knockout neurons, to review synaptic anchoring utilizing an alternative approach. Particularly, we’ve saved conditional AMPAR knockout neurons (in slices from Gria1-3 floxed rodents), with recombinant GluA1 ± NTD and GluA2Q ± NTD subunits, evaluating synaptic current amplitudes to native neurons (not experiencing knockout or save) in paired tracks (see Figure 6 from the revised manuscript). By using this approach, we visit a obvious dependence of GluA2 on its NTD for synaptic anchoring, deletion which reduces current save by roughly 50% . While GluA1 ΔNTD has the capacity to save synaptic currents even without the native receptors (compare Figure 6B2 with Figure 5D1 from the revised manuscript), save was similarly impaired on comparison to wild-type GluA1. Interestingly, GluA2 save was substantially greater than GluA1, mirroring the variations in RI seen on overexpression.
Taken together these extra data sets show our overexpression approach is neither excessive, nor dramatically non-physiological so we think that the extra insights we’ve acquired adds considerable strength to the original conclusions.
Another major concern pertains to the mechanism through which overexpression of GluA2Q increases evoked synaptic transmission. Based on the data presented, the mEPSC amplitude and frequency aren’t bigger when compared with control, as the single funnel conductance is. To keep the mEPSC amplitude despite greater single funnel conductance with GluA2Q overexpression, less AMPA receptors could be likely to show up per synapse. Is that this the situation or perhaps is there another reason behind these puzzling findings?
We agree that it is really an important point, yet it’s hard to draw such conclusions from mEPSC data. Concerning the mechanism for elevated evoked transmission, overexpression of GluA2Q substantially boosts the EPSC amplitude (Figure 1D from the original manuscript), which will probably be predominantly mediated through the alternation in synaptic funnel conductance as continues to be mentioned within the revised manuscript (Discussion, sixth paragraph).
3) We’ve investigated NTD-dependent AMPAR anchoring utilizing a save strategy in AMPAR conditional knockout neurons, to review synaptic anchoring utilizing an alternative approach. Particularly, we’ve saved conditional AMPAR knockout neurons (in slices from Gria1-3 floxed rodents), with recombinant GluA1 ± NTD and GluA2Q ± NTD subunits, evaluating synaptic current amplitudes to native neurons (not experiencing knockout or save) in paired tracks (see Figure 6 from the revised manuscript). By using this approach, we visit a obvious dependence of GluA2 on its NTD for synaptic anchoring, deletion which reduces current save by roughly 50% . While GluA1 ΔNTD has the capacity to save synaptic currents even without the native receptors (compare Figure 6B2 with Figure 5D1 from the revised manuscript), save was similarly impaired on comparison to wild-type GluA1. Interestingly, GluA2 save was substantially greater than GluA1, mirroring the variations in RI seen on overexpression.
Taken together these extra data sets show our overexpression approach is neither excessive, nor dramatically non-physiological so we think that the extra insights we’ve acquired adds considerable strength to the original conclusions.
Another major concern pertains to the mechanism through which overexpression of GluA2Q increases evoked synaptic transmission. Based on the data presented, the mEPSC amplitude and frequency aren’t bigger when compared with control, as the single funnel conductance is. To keep the mEPSC amplitude despite greater single funnel conductance with GluA2Q overexpression, less AMPA receptors could be likely to show up per synapse. Is that this the situation or perhaps is there another reason behind these puzzling findings?
We agree that it is really an important point, yet it’s hard to draw such conclusions from mEPSC data. Concerning the mechanism for elevated evoked transmission, overexpression of GluA2Q substantially boosts the EPSC amplitude (Figure 1D from the original manuscript), which will probably be predominantly mediated through the alternation in synaptic funnel conductance as continues to be mentioned within the revised manuscript (Discussion, sixth paragraph).
However, mEPSC tracks tend to be more hard to decipher because of the event recognition limit. Many occasions occur, which aren’t detected because they are underneath the recognition limit. Actual increases in the event amplitude allows formerly sub-threshold occasions to become detected, growing the mEPSC frequency, the average amplitude will probably be unaffected because of the contribution of more small, formerly undetected occasions towards the average value. Decreases in the event amplitude can similarly affect mEPSC frequency. This caveat was mentioned within the Methods portion of the original manuscript but has been more directly emphasized the revised manuscript (subsection “GluA2 ΔNTD reduces spontaneous transmission”, second paragraph and subsection “Electrophysiology”, first paragraph). Such interplay between mEPSC amplitude and frequency continues to be highlighted formerly by multiple laboratories (Lu et al. Neuron 2009 Rumbaugh et al. PNAS 2006).
As the improvement in mEPSC frequency between untransfected and GluA2Q expressing cells isn’t statistically significant since these data are highly variable (untransfected frequency varies from .14 to at least one.08 Hz), both apparent elevation in mEPSC frequency (Figure 2B from the original manuscript) by GluA2Q and the rise in amplitude of huge mEPSCs apparent in Figure 2A3 (original manuscript)are suggestive of a rise in event amplitude that won’t be symbolized through the mean event amplitude (Figure 2A2 from the original manuscript), due to the limitations described above.
We don’t believe that mEPSC information is sufficiently informative to take a position on such specific information on synaptic AMPAR content, since interplay between event amplitude and frequency, with evoked EPSC data being more reliably construed for this function. As formerly mentioned, evoked EPSCs shows a obvious rise in amplitude, which we feel convincingly argues against an extreme reduction in synaptic receptor figures. For that reasons detailed above we assert caution to not over-interpret mEPSC data, however our mEPSC dataset unambiguously shows variations between GluA2Q and GluA2Q ΔNTD for amplitude and frequency, strongly supporting our conclusions regarding the requirement of the NTD in synaptic anchoring.
https://doi.org/10.7554/eLife.23024.020
Resourse: https://elifesciences.org/articles/
