Sunrise in the synapse: the fmrp mrnp shaping the synaptic interface – sciencedirect
Contents
- Defining FMRP Target mRNAs In Situ
- A Molecular Model for FMRP Repression of Synaptic mRNAs
- Fragile X Spines: An Abnormal Phenotype Supplying Understanding of Synaptic Function
- A Task-Dependent Role for FMRP in Dendritic Spines
- FMRP in Synaptic Plasticity: Where Spine Structure May Directly Regulate Function
- FMRP Upkeep of the Cytoskeletal Architecture
- Defining FMRP Target mRNAs In Situ
- A Molecular Model for FMRP Repression of Synaptic mRNAs
- Fragile X Spines: An Abnormal Phenotype Supplying Understanding of Synaptic Function
- A Task-Dependent Role for FMRP in Dendritic Spines
- FMRP in Synaptic Plasticity: Where Spine Structure May Directly Regulate Function
- FMRP Upkeep of the Cytoskeletal Architecture
- A Job for FMRP within the Axon
- Conclusions
- 2-Minute Neuroscience: Synaptic Transmission
Defining FMRP Target mRNAs In Situ
An natural disadvantage of prior microarray approaches is the necessity to extract mRNA from cells, tissues, or fractions thereof. To know FMRP’s function in neurons, it might be important to test whether these FMRP-mRNA interactions exist in dendrites. It has now been accomplished, in another technical innovation in the Eberwine laboratory. Within the preceding issue of Neuron, investigators in the Ebwerwine and Greenough laboratories describe the event and use of a singular technology for that identification of mRNA targets from the FMRP in situ (Miyashiro et al., 2003). The process is known as “APRA,” for Antibody Positioned RNA Amplification, that involves coupling an oligonucleotide primer to some monoclonal antibody that binds to FMRP in fixed cells, positioning the primer for in situ transcription from the mRNAs which are presumably within the FMRP ribonucleoprotein complex. Following extraction of cDNA from cells, another strand cDNA synthesis is conducted, adopted by aRNA amplification. Labeled RNA probes generated in this way are hybridized to cDNA macroarrays, and positive cDNAs will be evaluated by filter binding and Ultra violet crosslinking. Roughly 60% from the APRA-defined mRNAs were proven to directly affiliate with FMRP. Of note, most of the FMRP-mRNA targets identified weren’t revealed through the previous study utilizing a co-IP method (Brown et al., 2001) and the other way around. The variations between your FMRP-mRNA targets identified between these studies is probably because there’s little overlap within the genes symbolized within the Affymetrix microarray used in the last study using the cDNA macroarrays used here. However, when one examines only individuals genes that overlap between these arrays, there have been several mRNA targets which were positively identified both in studies. Another essential distinction between the studies may be the use here of neuronally enriched mRNA from cultured neurons, instead of using whole brain for that co-IP analysis or lymphoblastoid cells for translational defects (Brown et al., 2001).
In Miyashiro et al., several methods were utilised to evaluate the expression and subcellular localization of FMRP-connected mRNAs as well as their encoded proteins. The lack of FMRP led to three distinct mRNA expression patterns which include reduced specific mRNA levels in hippocampal and cerebellar neurons in vivo, reduced mRNA levels and lack of dendritic localization, with no alternation in mRNA levels or localization. In the protein level, variations within the relative abundance of countless FMRP targets were noticed in total brain lysate and synaptosomal fractions, suggesting impairment in dendritic or synaptic targeting.
In conclusion, this research describes a cutting-edge approach which has defined many FMRP target mRNAs and suggests an extremely diverse role for FMRP in mRNA regulation. A significant question to become addressed is whether or not these mRNAs show altered translational regulation at synapses in Fmr1 knockout rodents.
A Molecular Model for FMRP Repression of Synaptic mRNAs
Evidence for any purpose of FMRP in translational repression in the synapse continues to be acquired inside a recent Cell paper in the laboratory of Bagni et al. (Zalfa et al., 2003). FMRP was proven to stay in an intricate with several mRNAs, including MAP1B, a known FMRP target, and CaMKIIα and Arc, two dendritically localized mRNAs (Steward and Schuman, 2001). The observation that CaMKIIα and Arc mRNA were detected in FMRP antibody precipitates is surprising, because these mRNAs weren’t detected by from the micoarray studies discussed above. In brain fractions of Fmr1 knockout rodents, the amount of those FMRP-connected mRNAs were elevated in polyribosome fractions. Elevated protein levels were also noticed in whole-brain and/or synaptosomal fractions, supplying further support for any role of FMRP in translational repression at synapses.
A fascinating twist within this study is the fact that FMRP wasn’t proven to have interaction directly using these mRNAs, as discussed above, but instead not directly via BC1 RNA (Figure 2D), a brief noncoding transcript that’s rich in brain as well as transported into dendrites (Muslimov et al., 1997). Recent focus on BC1 in the Tiedge laboratory has revealed its role in translational repression by inhibiting formation from the 48S preinitiation complex. Additionally, BC1 RNA was connected with poly (A) binding protein and eIF4A, each of which were enriched synaptically (Wang et al., 2002). Within the study by Zalfa et al., they reveal that BC1 RNA was connected with FMRP and hypothesize that BC1 RNA forms intermolecular base pairing with specific mRNAs, suggesting it functions like a bridge between FMRP and particular mRNAs (i.e., MAP1B). Data meant for this model were observations that the antisense oligonucleotide towards the BC1 sequence, recommended to base pair with MAP1B mRNA, could hinder the association of MAP1B mRNA with FMRP.
This research provides important new observations that demonstrate the translation of specific mRNAs is upregulated even without the FMRP, possibly resulting in excessive local protein synthesis in the synapse and in line with the purpose for FMRP in translational repression (Laggerbauer et al., 2001). However, it had been unclear why FMRP wasn’t detected in polyribosome fractions on sucrose gradients, as continues to be observed by others (Feng et al., 1997).
To supply further support for that model suggested (Zalfa et al., 2003), it will likely be vital that you characterize more exactly the mechanism of BC1-facilitated FMRP repression by validation from the base-pairing theory and molecular analysis of methods a BC1/FMRP complex may regulate, for instance, translation initiation.
Fragile X Spines: An Abnormal Phenotype Supplying Understanding of Synaptic Function
Just how can altered synaptic mRNA translation cause defects in synaptic morphology noticed in Fragile X Syndrome? Spine “dysmorphogenesis” was explained Purpura (1974), who demonstrated that dendritic spines of MR patients were longer, thinner, and less in number than individuals of asymptomatic individuals. Though lengthy and thin, spines of Fragile X people are hyperabundant (reviewed in Greenough et al. 2001, Fiala et al. 2002). Many investigators are analyzing whether morphology or density of dendritic spines underlies mechanisms of learning and memory impaired in Fragile X.
Immature spine morphology continues to be reported in slice cultures from the barrel cortex of Fmr1 knockout rodents throughout a window of 7–14 days (Nimchinsky et al., 2001). This window coincides using the critical duration of plasticity for your structure, a time period of intense permanent functional alteration as a result of stimuli. In comparison, in dissociated 3-week-old hippocampal neurons of Fmr1-deficient rodents, abnormally short and occasional-density synaptic spines were observed (Braun and Segal, 2000.) Reduced functional contacts within the hippocampus supported these changes, as did reduced excitatory synaptic currents. The transient phenotype noticed in these two studies, gone soon after the structures exit their particular critical periods, may point toward a vital role for FMRP in mediating plastic occasions. Is that this signature spine morphology of Fragile X as a result of compensatory mechanism from the cell (Fiala et al., 2002) or a result of FMRP loss? Presuming an immediate role for FMRP, two processes, synaptogenesis and spine maintenance, are implicated in Forex syndrome, a 1-protein disease, suggesting a dual role with this single protein.
A Task-Dependent Role for FMRP in Dendritic Spines
Since FMRP continues to be proven to localize to spines via immunoelectron microscopy (Feng et al., 1997), a task-dependent role for FMRP expression and localization continues to be searched for. Several groups utilized cortical synaptosomal formulations to show that FMRP levels increase as a result of both barrel cortex stimulation in vivo and mGluR activation in vitro. Furthermore, polyribosomes fractionated on sucrose gradients demonstrate a shift once they are probed for Fmr1 mRNA publish mGluR stimulation (reviewed, references reported in Greenough et al., 2001). The second study shows that Fmr1 mRNA exists and converted in the synapse and offers yet another method for FMRP localization and concentration in the synapse within an activity-dependent manner. Outdoors from the cortex, immunohistochemical studies within the hippocampus, a structure essential in learning and memory, have proven that FMRP levels can increase after rearing rodents in complex environments (for review, see Greenough et al., 2001).
FMRP in Synaptic Plasticity: Where Spine Structure May Directly Regulate Function
Due to the activity-dependence of FMRP, and also the MR that results with no protein, many groups examine different types of lengthy-term plasticity. A thrilling recent study has reported enhanced mGluR-dependent lengthy-term depression (Limited) in hippocampal slices from Fmr1 knockout rodents (Huber et al., 2002 see also Figure 2A). These investigators formerly demonstrated that group 1 mGluR5 signaling caused a protein synthesis-dependent type of Limited that relies upon postsynaptic protein synthesis and involves internalization of AMPA and NMDA receptors (Snyder et al., 2001). Bear and Huber advise a outcomes of enhanced mGluR-dependent Limited and spine elongation one possibility is the fact that activation of mGluR receptors can result in a nearby increase of calcium from internal stores, that has been connected with lengthening of dendritic spines (Vanderklish and Edelman, 2002). They claim that FMRP may negatively regulate or repress mRNAs involved with mGluR-dependent Limited. Even without the FMRP, excessive Limited ensues, which can be a prelude to synapse elimination and connected spine defects. One model is the fact that excessive synapse elimination, brought on by elevated Limited, results in a rise in filopodia, that is a reaction to losing spines.
Though impaired LTP is not reported within the hippocampus, Li et al. (2002) used tetanic stimulation to induce LTP within the cortex, cerebellum, and hippocampus of Fmr1-deficient rodents at 8–10 days. They based in the cortex there was both reduced cortical LTP along with a reduced quantity of GluR1 receptors. While no microarray research has indicated direct alterations in GluR1 mRNA levels in Fmr1-deficient creatures, it’s possible that GluR1 regulation occurs through other proteins whose mRNAs are differentially controlled within the knockout.
FMRP Upkeep of the Cytoskeletal Architecture
Other roles for FMRP regulating spine morphology are recommended by its binding interactions with proteins and mRNAs that shape cytoskeletal architecture. First, FMRP binds to Cytoplasmic FMRP Interacting Protein 1 (CYFIP1) (for review, see Bardoni and Mandel, 2002), which, consequently, binds to Rac-1, part of the subgroup from the Ras superfamily of GTPases. These GTPases are “switches” that regulate the actin cytoskeleton and therefore are implicated in dendritic modeling and remodeling (Figure 2B). When Rac-1 is constitutively active, it’s connected by having an abnormal rise in spine density, a known phenotypic abnormality in Fragile X Syndrome. Rac-1 is regarded as active in the maturation and upkeep of dendritic spines (Nakayama et al., 2000).
Defining FMRP Target mRNAs In SituAn natural disadvantage of prior microarray approaches is the necessity to extract mRNA from cells, tissues, or fractions thereof. To know FMRP’s function in neurons, it might be important to test whether these FMRP-mRNA interactions exist in dendrites. It has now been accomplished, in another technical innovation in the Eberwine laboratory. Within the preceding issue of Neuron, investigators in the Ebwerwine and Greenough laboratories describe the event and use of a singular technology for that identification of mRNA targets from the FMRP in situ (Miyashiro et al., 2003). The process is known as “APRA,” for Antibody Positioned RNA Amplification, that involves coupling an oligonucleotide primer to some monoclonal antibody that binds to FMRP in fixed cells, positioning the primer for in situ transcription from the mRNAs which are presumably within the FMRP ribonucleoprotein complex. Following extraction of cDNA from cells, another strand cDNA synthesis is conducted, adopted by aRNA amplification. Labeled RNA probes generated in this way are hybridized to cDNA macroarrays, and positive cDNAs will be evaluated by filter binding and Ultra violet crosslinking. Roughly 60% from the APRA-defined mRNAs were proven to directly affiliate with FMRP. Of note, most of the FMRP-mRNA targets identified weren’t revealed through the previous study utilizing a co-IP method (Brown et al., 2001) and the other way around. The variations between your FMRP-mRNA targets identified between these studies is probably because there’s little overlap within the genes symbolized within the Affymetrix microarray used in the last study using the cDNA macroarrays used here. However, when one examines only individuals genes that overlap between these arrays, there have been several mRNA targets which were positively identified both in studies. Another essential distinction between the studies may be the use here of neuronally enriched mRNA from cultured neurons, instead of using whole brain for that co-IP analysis or lymphoblastoid cells for translational defects (Brown et al., 2001).
In Miyashiro et al., several methods were utilised to evaluate the expression and subcellular localization of FMRP-connected mRNAs as well as their encoded proteins. The lack of FMRP led to three distinct mRNA expression patterns which include reduced specific mRNA levels in hippocampal and cerebellar neurons in vivo, reduced mRNA levels and lack of dendritic localization, with no alternation in mRNA levels or localization. In the protein level, variations within the relative abundance of countless FMRP targets were noticed in total brain lysate and synaptosomal fractions, suggesting impairment in dendritic or synaptic targeting.
In conclusion, this research describes a cutting-edge approach which has defined many FMRP target mRNAs and suggests an extremely diverse role for FMRP in mRNA regulation. A significant question to become addressed is whether or not these mRNAs show altered translational regulation at synapses in Fmr1 knockout rodents.
A Molecular Model for FMRP Repression of Synaptic mRNAs
Evidence for any purpose of FMRP in translational repression in the synapse continues to be acquired inside a recent Cell paper in the laboratory of Bagni et al. (Zalfa et al., 2003). FMRP was proven to stay in an intricate with several mRNAs, including MAP1B, a known FMRP target, and CaMKIIα and Arc, two dendritically localized mRNAs (Steward and Schuman, 2001). The observation that CaMKIIα and Arc mRNA were detected in FMRP antibody precipitates is surprising, because these mRNAs weren’t detected by from the micoarray studies discussed above. In brain fractions of Fmr1 knockout rodents, the amount of those FMRP-connected mRNAs were elevated in polyribosome fractions. Elevated protein levels were also noticed in whole-brain and/or synaptosomal fractions, supplying further support for any role of FMRP in translational repression at synapses.
A fascinating twist within this study is the fact that FMRP wasn’t proven to have interaction directly using these mRNAs, as discussed above, but instead not directly via BC1 RNA (Figure 2D), a brief noncoding transcript that’s rich in brain as well as transported into dendrites (Muslimov et al., 1997). Recent focus on BC1 in the Tiedge laboratory has revealed its role in translational repression by inhibiting formation from the 48S preinitiation complex. Additionally, BC1 RNA was connected with poly (A) binding protein and eIF4A, each of which were enriched synaptically (Wang et al., 2002). Within the study by Zalfa et al., they reveal that BC1 RNA was connected with FMRP and hypothesize that BC1 RNA forms intermolecular base pairing with specific mRNAs, suggesting it functions like a bridge between FMRP and particular mRNAs (i.e., MAP1B). Data meant for this model were observations that the antisense oligonucleotide towards the BC1 sequence, recommended to base pair with MAP1B mRNA, could hinder the association of MAP1B mRNA with FMRP.
This research provides important new observations that demonstrate the translation of specific mRNAs is upregulated even without the FMRP, possibly resulting in excessive local protein synthesis in the synapse and in line with the purpose for FMRP in translational repression (Laggerbauer et al., 2001). However, it had been unclear why FMRP wasn’t detected in polyribosome fractions on sucrose gradients, as continues to be observed by others (Feng et al., 1997).

To supply further support for that model suggested (Zalfa et al., 2003), it will likely be vital that you characterize more exactly the mechanism of BC1-facilitated FMRP repression by validation from the base-pairing theory and molecular analysis of methods a BC1/FMRP complex may regulate, for instance, translation initiation.
Fragile X Spines: An Abnormal Phenotype Supplying Understanding of Synaptic Function
Just how can altered synaptic mRNA translation cause defects in synaptic morphology noticed in Fragile X Syndrome? Spine “dysmorphogenesis” was explained Purpura (1974), who demonstrated that dendritic spines of MR patients were longer, thinner, and less in number than individuals of asymptomatic individuals. Though lengthy and thin, spines of Fragile X people are hyperabundant (reviewed in Greenough et al. 2001, Fiala et al. 2002). Many investigators are analyzing whether morphology or density of dendritic spines underlies mechanisms of learning and memory impaired in Fragile X.
Immature spine morphology continues to be reported in slice cultures from the barrel cortex of Fmr1 knockout rodents throughout a window of 7–14 days (Nimchinsky et al., 2001). This window coincides using the critical duration of plasticity for your structure, a time period of intense permanent functional alteration as a result of stimuli. In comparison, in dissociated 3-week-old hippocampal neurons of Fmr1-deficient rodents, abnormally short and occasional-density synaptic spines were observed (Braun and Segal, 2000.) Reduced functional contacts within the hippocampus supported these changes, as did reduced excitatory synaptic currents. The transient phenotype noticed in these two studies, gone soon after the structures exit their particular critical periods, may point toward a vital role for FMRP in mediating plastic occasions. Is that this signature spine morphology of Fragile X as a result of compensatory mechanism from the cell (Fiala et al., 2002) or a result of FMRP loss? Presuming an immediate role for FMRP, two processes, synaptogenesis and spine maintenance, are implicated in Forex syndrome, a 1-protein disease, suggesting a dual role with this single protein.
A Task-Dependent Role for FMRP in Dendritic Spines
Since FMRP continues to be proven to localize to spines via immunoelectron microscopy (Feng et al., 1997), a task-dependent role for FMRP expression and localization continues to be searched for. Several groups utilized cortical synaptosomal formulations to show that FMRP levels increase as a result of both barrel cortex stimulation in vivo and mGluR activation in vitro. Furthermore, polyribosomes fractionated on sucrose gradients demonstrate a shift once they are probed for Fmr1 mRNA publish mGluR stimulation (reviewed, references reported in Greenough et al., 2001). The second study shows that Fmr1 mRNA exists and converted in the synapse and offers yet another method for FMRP localization and concentration in the synapse within an activity-dependent manner. Outdoors from the cortex, immunohistochemical studies within the hippocampus, a structure essential in learning and memory, have proven that FMRP levels can increase after rearing rodents in complex environments (for review, see Greenough et al., 2001).
FMRP in Synaptic Plasticity: Where Spine Structure May Directly Regulate Function
Due to the activity-dependence of FMRP, and also the MR that results with no protein, many groups examine different types of lengthy-term plasticity. A thrilling recent study has reported enhanced mGluR-dependent lengthy-term depression (Limited) in hippocampal slices from Fmr1 knockout rodents (Huber et al., 2002 see also Figure 2A). These investigators formerly demonstrated that group 1 mGluR5 signaling caused a protein synthesis-dependent type of Limited that relies upon postsynaptic protein synthesis and involves internalization of AMPA and NMDA receptors (Snyder et al., 2001). Bear and Huber advise a outcomes of enhanced mGluR-dependent Limited and spine elongation one possibility is the fact that activation of mGluR receptors can result in a nearby increase of calcium from internal stores, that has been connected with lengthening of dendritic spines (Vanderklish and Edelman, 2002). They claim that FMRP may negatively regulate or repress mRNAs involved with mGluR-dependent Limited. Even without the FMRP, excessive Limited ensues, which can be a prelude to synapse elimination and connected spine defects. One model is the fact that excessive synapse elimination, brought on by elevated Limited, results in a rise in filopodia, that is a reaction to losing spines.
Though impaired LTP is not reported within the hippocampus, Li et al. (2002) used tetanic stimulation to induce LTP within the cortex, cerebellum, and hippocampus of Fmr1-deficient rodents at 8–10 days. They based in the cortex there was both reduced cortical LTP along with a reduced quantity of GluR1 receptors. While no microarray research has indicated direct alterations in GluR1 mRNA levels in Fmr1-deficient creatures, it’s possible that GluR1 regulation occurs through other proteins whose mRNAs are differentially controlled within the knockout.
FMRP Upkeep of the Cytoskeletal Architecture
Other roles for FMRP regulating spine morphology are recommended by its binding interactions with proteins and mRNAs that shape cytoskeletal architecture. First, FMRP binds to Cytoplasmic FMRP Interacting Protein 1 (CYFIP1) (for review, see Bardoni and Mandel, 2002), which, consequently, binds to Rac-1, part of the subgroup from the Ras superfamily of GTPases. These GTPases are “switches” that regulate the actin cytoskeleton and therefore are implicated in dendritic modeling and remodeling (Figure 2B). When Rac-1 is constitutively active, it’s connected by having an abnormal rise in spine density, a known phenotypic abnormality in Fragile X Syndrome. Rac-1 is regarded as active in the maturation and upkeep of dendritic spines (Nakayama et al., 2000).
Another candidate role for FMRP altering synaptic structure originates from recent work on the Drosophila NMJ. Futsch rearranges cytoskeletal loops that form during division from the synaptic bouton. The sunshine chain of MAP1B, the mammalian homolog to Futsch, binds actin stress fibers in vivo (Togel et al., 1998). Within the dfmr1 mutant, there’s a rise in growth and branching as well as an overall rise in bouton number, by as much as 50%, that appears to derive from elevated Futsch expression (Gao, 2002). Evoked synaptic transmission seemed to be elevated within the dfmr1 mutant NMJ. In comparison, overexpression of dFMR1 both in pre- and postsynaptic compartments results in a reduction in bouton number. It had been figured that synaptic structure and performance is controlled by dFMR1 repression of Futsch mRNA (Gao, 2002). Once more, FMRP is from the structural architecture from the synapse and it is function.
A Job for FMRP within the Axon
Observations of axonal defects in dfmr1 null mutants advise a possible local function for FMRP within the regulating axonal outgrowth and/or presynaptic structure. Indeed, it had been proven by immunofluorescence (Zhang et al., 2001) that dFMR1 is localized to axons in normal flies. It’s possible the abnormal phenotypes really are a direct results of the lack of controlled Futsch mRNA translation by dFMR1 in axons. Observations of mRNA localization and translation in axons happen to be given new attention this is not a phenomenon limited to invertebrates (Guiditta et al., 2002). It might be exciting to recognize a particular function for FMRP in axons.
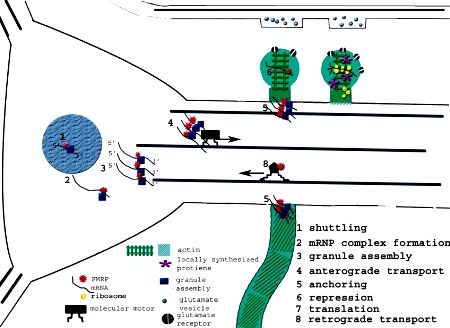
Conclusions
FMRP, a renaissance protein, seems to possess multifarious functions: repressor, shuttle, adaptor, and transporter. FMRP is not a part of an elusive mRNP, because so many interesting specific mRNA targets and connected mRNAs happen to be identified. The task would be to characterize the molecular mechanism(s) of methods FMRP may regulate local mRNA translation as a result of synaptic signaling pathways involved with lengthy-term plasticity. One apparent course is to locate if the specific defects in spine morphology and/or synaptic plasticity discussed within this review could be proven to become determined by altered translation of FMRP-connected mRNAs, which there are lots of interesting candidates to now study.
Resourse: https://sciencedirect.com/science/article/pii/
