Ip technique is a difficult balanced exercise for pharmaceuticals – ipwatchdog.com
Contents
- Timeline and price of drug development
- Patent or trade secret?
- Timeline and price of drug development
- Patent or trade secret?
- Patent extensions
- Data exclusivity
- Waiting to patent specific details
- A difficult balanced exercise
 The twenty years of protection afforded with a patent is supposed to promote innovation by permitting inventors an opportunity to recoup development costs and derive an income using their efforts. However, within the pharmaceutical industry, the sensible time period of protection is frequently substantially shorter since acquiring a patent is simply one piece—albeit a vital one–of getting a medication to promote.
The twenty years of protection afforded with a patent is supposed to promote innovation by permitting inventors an opportunity to recoup development costs and derive an income using their efforts. However, within the pharmaceutical industry, the sensible time period of protection is frequently substantially shorter since acquiring a patent is simply one piece—albeit a vital one–of getting a medication to promote.
Another piece is U.S. Federal Drug Administration (Food and drug administration) approval. Unlike other products, all drugs and medicines are susceptible to a regulatory review period before they may be offered within the U.S. – which process can consume a significant part of a drug’s patent term.
Timeline and price of drug development
It requires typically ten to fifteen many years to create a new drug – a procedure it is not only time-consuming but costly. The Tufts Center for study regarding Drug Development’s 2016 study discovered that the typical R&D costs of the drug are near to $2.6 billion before Food and drug administration approval is received, while increasing to almost $2.9 billion with publish-approval R&D.
When a brand new drug reaches the stage where it’s ready for clinical testing on humans, a considerable investment was already made. The pharmaceutical company has seen the drug through typically someone to six many years of compound discovery, synthesis, purification and frequently, limited animal testing. That is why most pharmaceutical companies affect patent their drugs throughout the research stage, before incurring the even-greater price of human numerous studies.
Food and drug administration approval is needed for that three stages of numerous studies, in the finish which — presuming the drug remains a practical product — your final New Drug Application (NDA) is filed using the Food and drug administration. Once the NDA qualifies can the organization begin selling the medication. At this time, the typical period of time left around the patent is simply 11.five years.
Patent or trade secret?
While acquiring a patent is easily the most apparent method to safeguard a company’s IP, filling a patent application presents two major trouble for the pharmaceutical industry:
- It starts the 20-year patent clock ticking (i.e. when the patent is granted, protection begins in the filing date).
- The invention which the brand new drug relies is uncovered to competitors, since patent applications are public.
 The twenty years of protection afforded with a patent is supposed to promote innovation by permitting inventors an opportunity to recoup development costs and derive an income using their efforts. However, within the pharmaceutical industry, the sensible time period of protection is frequently substantially shorter since acquiring a patent is simply one piece—albeit a vital one–of getting a medication to promote.
The twenty years of protection afforded with a patent is supposed to promote innovation by permitting inventors an opportunity to recoup development costs and derive an income using their efforts. However, within the pharmaceutical industry, the sensible time period of protection is frequently substantially shorter since acquiring a patent is simply one piece—albeit a vital one–of getting a medication to promote.
Another piece is U.S. Federal Drug Administration (Food and drug administration) approval. Unlike other products, all drugs and medicines are susceptible to a regulatory review period before they may be offered within the U.S. – which process can consume a significant part of a drug’s patent term.
Timeline and price of drug development
It requires typically ten to fifteen many years to create a new drug – a procedure it is not only time-consuming but costly. The Tufts Center for study regarding Drug Development’s 2016 study discovered that the typical R&D costs of the drug are near to $2.6 billion before Food and drug administration approval is received, while increasing to almost $2.9 billion with publish-approval R&D.
When a brand new drug reaches the stage where it’s ready for clinical testing on humans, a considerable investment was already made. The pharmaceutical company has seen the drug through typically someone to six many years of compound discovery, synthesis, purification and frequently, limited animal testing. That is why most pharmaceutical companies affect patent their drugs throughout the research stage, before incurring the even-greater price of human numerous studies.
Food and drug administration approval is needed for that three stages of numerous studies, in the finish which — presuming the drug remains a practical product — your final New Drug Application (NDA) is filed using the Food and drug administration. Once the NDA qualifies can the organization begin selling the medication. At this time, the typical period of time left around the patent is simply 11.five years.
Patent or trade secret?
While acquiring a patent is easily the most apparent method to safeguard a company’s IP, filling a patent application presents two major trouble for the pharmaceutical industry:
- It starts the 20-year patent clock ticking (i.e. when the patent is granted, protection begins in the filing date).
- The invention which the brand new drug relies is uncovered to competitors, since patent applications are public.
Consequently, some information mill embracing trade secrets. Based on the USPTO, trade secrets safeguard “information that may incorporate a formula, pattern, compilation, program, device, method, technique or process…[which] give[s] an chance to acquire a fiscal edge on competitors who don’t know or utilize it.
The time period of a trade secret’s indefinite, that makes it a beautiful option given the size of the Food and drug administration approval process. Within the U.S., a trade secret also provides a workaround for that recent limitations on patents involving laws and regulations of nature. However, counting on trade secrets is dangerous – if uncovered (whether purposely or accidentally), their protection evaporates.
Patent extensions
The proprietor of the pharmaceutical patent can use towards the USPTO to have an extension of the patent term to compensate for a few of the time lost towards the Food and drug administration approval process. However, the guidelines really are a bit complicated and extensions rarely equal the patent time a business may go through it’s lost. Listed here are the provisions to get extra time:
- Time into consideration for extension begins around the date the patent is disseminated.
- The utmost extension possible is equivalent to 1 / 2 of the Food and drug administration review period.
- The extension might not boost the effective patent term to greater than 14 years.
To determine the way the rules are applied, let’s tell you an average pharma company’s development process:
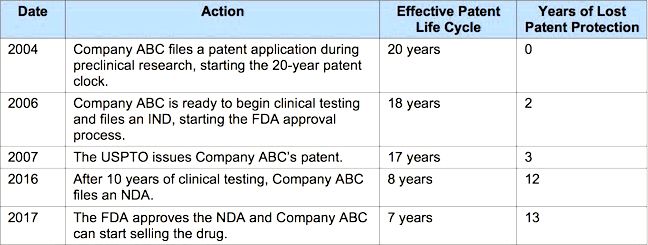 Now, let’s use the rules:
Now, let’s use the rules:
- Time into consideration comes from 2007 (date of patent issue) to 2017 (finish of regulatory review period). That’s ten years.
- Company ABC can obtain a maximum extension of five years (half of times into consideration).
- Extra time of five years would enhance the patent term to 12 years (since the effective patent existence cycle was lower to many years upon receipt of Food and drug administration approval). As this is under 14 years, what the law states would enable the maximum extension.
Data exclusivity
The Food and drug administration may grant a little more protection to add mass to biologic drugs – i.e. genetically-engineered medications according to living microorganisms – via 12-year “data exclusivity” periods. These periods grant the pharma company exclusive legal rights to the medical trial data so the data cannot be utilised by a duplicate-cat competitor to rapidly get yourself a patent for any cheaper “biosimilar” drug.
Waiting to patent specific details
Another strategy is to use for any drug’s patents in phases. A pharmaceutical company could decide initially to use just for patent protection from the drug’s underlying compound before numerous studies to provide very little away as you possibly can concerning the product. Later, they are able to seek additional, more specific — and revealing — “method of use” and “formulation” patents because they catch up with to final Food and drug administration approval.
A difficult balanced exercise
With the much cash on the line and competition to make money so fierce, a pharmaceutical’s monetization roadmap requires a effective IP technique for sufficient protection. It’s a difficult balanced exercise between secrecy, security and timing.
Resourse: https://ipwatchdog.com/2017/08/26/ip-strategy-tricky-balancing-pharmaceuticals/id=86948/
