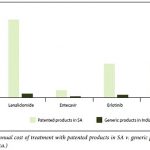
Bradley white-colored
Mylan Pharmaceuticals ULC:
Lead outdoors counsel to Mylan Pharmaceuticals ULC in patent litigation, prosecution along with other matters. Presently representing or effectively settled numerous complex pharmaceutical litigation matters with respect to Mylan with regards to products seeking market entry including TECTA® (Pantoprazole Mg), CIALIS® (Tadalafil), SENSIPAR® (Cinacalcet) and VIMOVO® (Naproxen and esomeprazole Mg).
Amgen Corporation et. al. v BHP Pharma ULC dba Mylan EPD T-420-19
AstraZeneca Canada Corporation. et al. v Mylan Pharmaceuticals ULC et al. T-336-15 (2017), T-184-19
Eli Lilly et al. v. Mylan Pharmaceuticals ULC T-1627-16
Takeda Pharmaceuticals et al. v. Mylan Pharmaceuticals ULC et al. T-1161-13 (2015) FC 751
Takeda Pharmaceuticals et al. v. Mylan Pharmaceuticals ULC et al. T-1451-11 (2015)
Amgen Canada Corporation. v Mylan Pharmaceuticals ULC et al. T-2056-14 (2015)
Amgen Canada Corporation. v. Mylan Pharmaceuticals ULC et al T-1584-17
(Federal Court File No. T-1584-17) SENSIPAR (Cinacalcet) Section 8 Damages
Novartis Pharmaceuticals v Mylan Pharmaceuticals ULC et al. T-365-14 (2014)
AstraZeneca et al. v Mylan Pharmaceuticals ULC et al. 2011 FC 312, 2012 FCA 109
Pfizer Canada Corporation et al. v Mylan Pharmaceuticals ULC et al. 2011 FC 547 2012 FCA 103
Janssen Corporation. v Mylan Pharmaceuticals ULC et al. T-1577-14 (2011)
Sanofi-Aventis v Mylan Pharmaceuticals ULC et al. T-1441-12 (2010)
Lundbeck Canada Corporation. v Mylan Pharmaceuticals ULC et al. (2009) 73 C.P.R. (fourth) 69 (F.C.T.D.) (2010) 88 C.P.R. (fourth) 325 (F.C.A.), SCC 34068
Teva Canada Limited:
Lead outdoors counsel to Teva Canada Limited in patent litigation, prosecution along with other matters. Presently representing or effectively settled complex pharmaceutical and general litigation matters with respect to Teva including with regards to CUBICIN® (Daptomycin), SPRYCEL® (Dasatinib Monohydrate) and VICTOZA® (Liraglutide).
Bristol-Myers Squibb Canada Co v. Teva Canada Limited T-1774-18
Bayer Corporation. and Bayer Ip GMBH v. Teva Canada Limited T-1960-18, T-1021-19.
Bayer Corporation. et al v. Teva Canada Limited T-1692-18
Novo Nordisk Canada Corporation. v. Teva Canada Limited. et al. T-727-18, T-65-19
Teva/Celltrion Healthcare:
Lead outdoors counsel to Teva/Celltrion in complex pharmaceutical litigation matters such as the biosimilars to RITUXAN® (Rituximab) and HERCEPTIN® (Trastuzumab).
F. Hoffmann-La Roche Limited v. Celltrion et al. T-1486-17, T-1487-17, T-1488-17, T-1489-17
Genentech, Corporation. et al. v. Celltrion Healthcare Co., Limited. T-1969-17, T-1970-17, T-1971-17
Samsung Bioepis:
Representing Samsung Bioepis in a number of significant patent litigation cases, such as the first situation introduced in Canada underneath the Patented Medicines (Notice of Compliance) Rules with regards to blockbuster biopharmaceutical ENBREL® (etanercept). Also symbolized Samsung in patent litigation proceedings regards to the world’s greatest drug HUMIRA® (adalimumab).
Abbvie Biotechnology et al v. Samsung Bioepis Co., Limited. and also the Minister of Health T-598-17, T-599-17, T-600-17, T-601-17, T-602-17, T-603-17, T-604-17, T-605-17
Amgen Canada Corporation. and Immunex Corporation v. Samsung Bioepis Co., Limited. and also the Minister of Health T-1283-15 (2014)
Standard Innovation Corporation:
Representing Standard Innovation Corporation (now WOW Tech) becoming world-wide coordinating litigation and IP counsel, such as the following cases:
Opposition Proceedings according of European Patent No. Air 1 824 440 within the European Patent Office
Appeal in the US Worldwide Trade Commission, Analysis No. 337-TA-823, U . s . States Court of Appeals for that Federal Circuit, No. 2013-1582, Lelo Corporation. & Leloi AB v. Worldwide Trade Commission and Standard Innovation (US) Corp. & Standard Innovation Corporation
District Legal Action prior to the U . s . States District Court of Northern California 5:13-cv-01393-PSG: Lelo Corporation v. Standard Innovation (US) Corp and Standard Innovation Corporation
Ex Parte reexamination from the U . s . States Patent No. 7,931,605 (“the ‘605 Patent”)
Lead Canadian IP counsel to plain Innovation Corporation including in T-925-12: Standard Innovation Corporation v. Lelo AB et Lelo Corporation., 1531011 Ontario Corporation., Cana Worldwide Disbursing Corporation., Landco Import Worldwide Corporation. and TBMBM Corporation.
Two Inter Partes Review Re: Standard Innovation Corporation (Petitioner) v. Lelo Corporation. [Patent Owner Cases IPR 2014-00148 and IPR 2014-00907, Re: U . s . States Patent No. 7,749,178 (“the ‘178 Patent”)].
ratiopharm Corporation:
Abbott Laboratories v. ratiopharm Corporation. et al. (2004), 31 C.P.R. (4th) 321 (F.C.A.)
Abbott Laboratories v. ratiopharm Corporation. et al. (2005), 42 C.P.R. (4th) 20 and 121 (F.C.T.D.)
Mylan Pharmaceuticals ULC:
Lead outdoors counsel to Mylan Pharmaceuticals ULC in patent litigation, prosecution along with other matters. Presently representing or effectively settled numerous complex pharmaceutical litigation matters with respect to Mylan with regards to products seeking market entry including TECTA® (Pantoprazole Mg), CIALIS® (Tadalafil), SENSIPAR® (Cinacalcet) and VIMOVO® (Naproxen and esomeprazole Mg).
Amgen Corporation et. al. v BHP Pharma ULC dba Mylan EPD T-420-19
AstraZeneca Canada Corporation. et al. v Mylan Pharmaceuticals ULC et al. T-336-15 (2017), T-184-19
Eli Lilly et al. v. Mylan Pharmaceuticals ULC T-1627-16
Takeda Pharmaceuticals et al. v. Mylan Pharmaceuticals ULC et al. T-1161-13 (2015) FC 751
Takeda Pharmaceuticals et al. v. Mylan Pharmaceuticals ULC et al. T-1451-11 (2015)
Amgen Canada Corporation. v Mylan Pharmaceuticals ULC et al. T-2056-14 (2015)
Amgen Canada Corporation. v. Mylan Pharmaceuticals ULC et al T-1584-17
(Federal Court File No. T-1584-17) SENSIPAR (Cinacalcet) Section 8 Damages
Novartis Pharmaceuticals v Mylan Pharmaceuticals ULC et al. T-365-14 (2014)
AstraZeneca et al. v Mylan Pharmaceuticals ULC et al. 2011 FC 312, 2012 FCA 109
Pfizer Canada Corporation et al. v Mylan Pharmaceuticals ULC et al. 2011 FC 547 2012 FCA 103
Janssen Corporation. v Mylan Pharmaceuticals ULC et al. T-1577-14 (2011)
Sanofi-Aventis v Mylan Pharmaceuticals ULC et al. T-1441-12 (2010)
Lundbeck Canada Corporation. v Mylan Pharmaceuticals ULC et al. (2009) 73 C.P.R. (fourth) 69 (F.C.T.D.) (2010) 88 C.P.R. (fourth) 325 (F.C.A.), SCC 34068
Teva Canada Limited:
Lead outdoors counsel to Teva Canada Limited in patent litigation, prosecution along with other matters. Presently representing or effectively settled complex pharmaceutical and general litigation matters with respect to Teva including with regards to CUBICIN® (Daptomycin), SPRYCEL® (Dasatinib Monohydrate) and VICTOZA® (Liraglutide).
Bristol-Myers Squibb Canada Co v. Teva Canada Limited T-1774-18
Bayer Corporation. and Bayer Ip GMBH v. Teva Canada Limited T-1960-18, T-1021-19.
Bayer Corporation. et al v. Teva Canada Limited T-1692-18
Novo Nordisk Canada Corporation. v. Teva Canada Limited. et al. T-727-18, T-65-19
Teva/Celltrion Healthcare:
Lead outdoors counsel to Teva/Celltrion in complex pharmaceutical litigation matters such as the biosimilars to RITUXAN® (Rituximab) and HERCEPTIN® (Trastuzumab).
F. Hoffmann-La Roche Limited v. Celltrion et al. T-1486-17, T-1487-17, T-1488-17, T-1489-17
Genentech, Corporation. et al. v. Celltrion Healthcare Co., Limited. T-1969-17, T-1970-17, T-1971-17
Samsung Bioepis:
Representing Samsung Bioepis in a number of significant patent litigation cases, such as the first situation introduced in Canada underneath the Patented Medicines (Notice of Compliance) Rules with regards to blockbuster biopharmaceutical ENBREL® (etanercept). Also symbolized Samsung in patent litigation proceedings regards to the world’s greatest drug HUMIRA® (adalimumab).
Abbvie Biotechnology et al v. Samsung Bioepis Co., Limited. and also the Minister of Health T-598-17, T-599-17, T-600-17, T-601-17, T-602-17, T-603-17, T-604-17, T-605-17
Amgen Canada Corporation. and Immunex Corporation v. Samsung Bioepis Co., Limited. and also the Minister of Health T-1283-15 (2014)
Standard Innovation Corporation:
Representing Standard Innovation Corporation (now WOW Tech) becoming world-wide coordinating litigation and IP counsel, such as the following cases:
Opposition Proceedings according of European Patent No. Air 1 824 440 within the European Patent Office
Appeal in the US Worldwide Trade Commission, Analysis No. 337-TA-823, U . s . States Court of Appeals for that Federal Circuit, No. 2013-1582, Lelo Corporation. & Leloi AB v. Worldwide Trade Commission and Standard Innovation (US) Corp. & Standard Innovation Corporation
District Legal Action prior to the U . s . States District Court of Northern California 5:13-cv-01393-PSG: Lelo Corporation v. Standard Innovation (US) Corp and Standard Innovation Corporation
Ex Parte reexamination from the U . s . States Patent No. 7,931,605 (“the ‘605 Patent”)
Lead Canadian IP counsel to plain Innovation Corporation including in T-925-12: Standard Innovation Corporation v. Lelo AB et Lelo Corporation., 1531011 Ontario Corporation., Cana Worldwide Disbursing Corporation., Landco Import Worldwide Corporation. and TBMBM Corporation.
Two Inter Partes Review Re: Standard Innovation Corporation (Petitioner) v. Lelo Corporation. [Patent Owner Cases IPR 2014-00148 and IPR 2014-00907, Re: U . s . States Patent No. 7,749,178 (“the ‘178 Patent”)].
ratiopharm Corporation:
Abbott Laboratories v. ratiopharm Corporation. et al. (2004), 31 C.P.R. (4th) 321 (F.C.A.)
Abbott Laboratories v. ratiopharm Corporation. et al. (2005), 42 C.P.R. (4th) 20 and 121 (F.C.T.D.)
Abbott Laboratories v. ratiopharm Corporation. et al. (2005) 34 C.P.R. (4th) 468 (F.C.T.D.)
Novopharm Limited:
Janssen-Ortho Corporation. and Daiichi Pharmaceutical Co, Limited. v. Novopharm Limited. et al. 2006 FC 1333, (2006) 57 C.P.R. (4th) 6 and 58 (F.C.T.D.)
Janssen-Ortho Corporation. v. Novopharm Limited. et al. (2005), 39 C.P.R. (4th) 197 (F.C.A.)
Janssen-Ortho Corporation. v. Novopharm Limited. et al. (2005), 35 C.P.R (4th) 353 (F.C.T.D.), appeal ignored (2005), 40 C.P.R. (4TH) 1 (F.C.A.)
Other:
Serving as counsel to Munchkin Baby Canada, Limited. and it is U.S. affiliate within the defence of the Federal Court patent violation action concerning the defendants’ making and selling of cartridges for diaper pails. Angelcare Development Corporation. et al. v. Munchkin, Corporation et al T-151-16
Counsel to Skretting Canada Corporation. in letters rogatory proceedings relating an american patent dispute concerning means of raising fish. Marical, Corporation. v. Skretting Canada Corporation. SJM-51-2016
Nycomed v. Genpharm ULC et al. 2008 FC 330, (2008), 64 C.P.R. (4th) 388 (F.C.T.D.)

Resourse: https://osler.com/en/team/
Photoshop – Turn color to white – change the color of anything to white
Photoshop – Make a color object into a white object Turn a color object into a white object in Photoshop. This video shows a quick and easy way to turn a color …