Huntley lab
 Synapse structure and performance is continuously modified during development and throughout existence by experience, for example learning additional skills or developing new recollections. Such synaptic plasticity is thus crucial for normal thinking processes. Synaptic plasticity may also become maladaptive under conditions of brain, spine or peripheral nerve injuries, resulting in abnormal function or sensation. Dr. Huntley’s research concentrates on mechanisms of synaptic plasticity by which synaptic structure and performance are modified by experience, injuries or genetic mutation. Studies include: 1) the function from the cadherin group of synaptic adhesion proteins in synapse and circuit development, plasticity and repair 2) genes, molecules and mechanisms controlling aberrant corticostriatal circuit development and plasticity in autism and Parkinson’s disease models.
Synapse structure and performance is continuously modified during development and throughout existence by experience, for example learning additional skills or developing new recollections. Such synaptic plasticity is thus crucial for normal thinking processes. Synaptic plasticity may also become maladaptive under conditions of brain, spine or peripheral nerve injuries, resulting in abnormal function or sensation. Dr. Huntley’s research concentrates on mechanisms of synaptic plasticity by which synaptic structure and performance are modified by experience, injuries or genetic mutation. Studies include: 1) the function from the cadherin group of synaptic adhesion proteins in synapse and circuit development, plasticity and repair 2) genes, molecules and mechanisms controlling aberrant corticostriatal circuit development and plasticity in autism and Parkinson’s disease models.
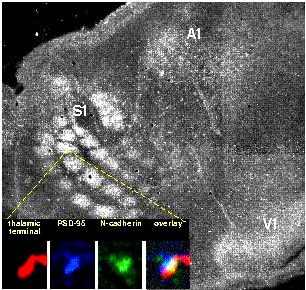
The synapse adhesion molecule neural (N)-cadherin is localized to developing thalamocortical synaptic junctional complexes. The bigger image is really a flattened, tangential section through layer IV of developing (P5) rat cortex that has been immunolabeled for N-cadherin. Terminal fields within the barrel (somatosensory) cortex (S1), auditory cortex (A1) and visual cortex (V1) are apparent within the patterns of N-cadherin labeling. The confocal microscope images proven within the inset verify that such terminal fields are N-cadherin labeled thalamocortical synapses, proven within the overlay through the codistributions of FluoroRuby-labeled ventrobasal thalamic afferent terminals (red), immunolabeling for PSD-95 (blue)–which is targeted in uneven postsynaptic densities–and N-cadherin (eco-friendly). Modified in the article by G.W. Huntley and D.L. Benson, “N-Cadherin at developing thalamocortical synapses offers an adhesion mechanism for that formation of somatotopically organized connections.” Journal of Comparative Neurology 407:453-471 (1999).
Resourse: http://labs.neuroscience.mssm.edu/project/huntley-lab/

