Need for the ip system in attempting compulsory licensing of pharmaceuticals: a mix-sectional analysis
Contents
- Good reputation for compulsory licensing of patents
- Good reputation for compulsory licensing of patents
- Role of compulsory licensing in patent systems and health systems
- Limitations from the study
- Need You – exbatalion lyric
Compulsory licensing might be from a government in situations in which the patent holder is either not while using patent or perhaps is not utilizing it adequately, including pharmaceuticals sought after that aren’t fully available. When governments make an effort to issue compulsory licensing or non-governmental entities request a government to issue compulsory licensing, the end result is frequently home loan business prices, like the situation of the development of generics on the market [16, 31]. Although compulsory licensing isn’t a new idea, it’s received considerable debates as patent holders aim to advance their political stances over ip legal rights to gain access to to medicines. Particularly, opponents of compulsory licensing reason that compulsory licensing could be diametrically against the part from the patent system.
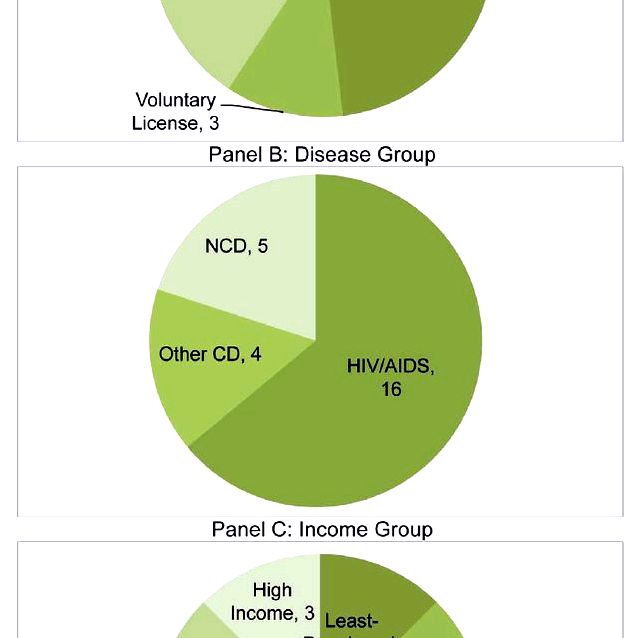
We designed this research to show the positive role from the matured ip system in attempting compulsory licensing. The issuance from the compulsory licensing of the patent needs a granted patent, the results of a functioning patent system. Therefore, a hyperlink exists between your compulsory licensing of patents and also the patent system. Within this study, we presented that using compulsory licensing is a valuable part from the patent system along with a legitimate tool for that government to make use of by demonstrating that compulsory licensing of pharmaceuticals is required particularly countries having a mature patent system.
To the understanding, this research is the first one to empirically analyse the connection between compulsory licensing and patent systems. We used a multivariate logistic model to regress the make an effort to issue compulsory licensing around the characteristics from the ip system (i.e., the PIPP index), controlling for macro context variables (i.e., region, population, earnings, and polity) in a country level. Interestingly, mature patent systems were positively connected with attempting compulsory licensing. How then might mature patent systems modify the issuance of compulsory licensing?
A brief history of compulsory licensing can help to explain these interesting results simply because they give a reason behind the presence of compulsory licensing inside a patent system. We’ll trace the origins from the compulsory licensing of patents in high-earnings countries and discuss interesting cases from Canada within the late 1980s.
Good reputation for compulsory licensing of patents
The next legislative cases within the 18-nineteenth century show high-earnings countries positively reviewed and introduced compulsory licensing to enhance the ip system and develop their very own industrial policy.
The compulsory licensing of patents was adopted through the compulsory licensing of copyrights [11]. In England, excessive cost as well as an inadequate supply by monopolies were acknowledged as a fiscal disease [32]. The Statute of Anne in 1710, referred to as world’s first copyright law, reflected this thought. Someone apart from who owns the copyright who determined that the book was over-priced could file a complaint against who owns the copyright to the court, along with a fine was enforced to who owns the copyright whether it violated the cost set through the court. Following a Statute of Anne, the U . s . States, particularly the condition of Connecticut, established the Copyright Act in 1783. The Copyright Act needed copyrighted books to become offered in a reasonable cost in sufficient quantities otherwise, it might be easy to file a complaint to the court, like the situation from the Statute of Anne. In addition, a legal court had the legal right to determine the amount and cost for that copyright owner when the copyright owner didn’t accept or rejected this type of request, a legal court had the legal right to possess the complainant print it. Therefore, the Copyright Act was evaluated like a legislative illustration of the world’s first system of compulsory licensing of copyrights [17].
The U . s . States, particularly the condition of Sc, established the Act for that Encouragement of Arts and Science, supplying rights and limitations enforced to authors (copyrights) in addition to inventors (patents) in 1784. The Act first devised compulsory licensing like a new remedy from the abuse from the patents when only invalidation from the patents existed. It ought to be noted the U . s . States have been more aggressive in working with patent abuse during the introduction of new industry. Similarly, England and Germany discussed compulsory licensing as measures to reduce the negative effects from the patent system and complement the shortcomings from the patent system in 1851 and 1853, correspondingly [18].
These trends favouring compulsory licensing in a variety of fields endured within the 1900s [5, 33]. The U . s . States invoked government use regularly to import pharmaceuticals within the 1960s. In addition, Canada utilized the compulsory licensing of pharmaceuticals not just for importation but in addition for local production to handle health expenditure [5, 34, 35]. For example, the Canadian government enacted compulsory licensing by amending the Patent Act to inspire local manufacturing from the pharmaceutical and market competition in 1923 [5]. In addition, there is public concern around the cost of pharmaceuticals which was considered as excessive through the 1960s. Because of the concern, the federal government made several amendments towards the Patent Act, which may have significant implications on patenting activities. Furthermore, the amendments permitted using compulsory licensing to fabricate or import pharmaceuticals which were limited to manufacturing before amendments [5]. The advantages of compulsory licensing are palpable in Canada, and numerous follow-on drugs have joined the marketplace.
However, the positive stance regarding compulsory licensing of high-earnings countries particularly altered within the last century, particularly throughout the negotiations for Journeys [36]. LMICs contended for the best to issue compulsory licensing for patented pharmaceuticals which were costly for his or her citizens, while high-earnings countries, usually affected by the pharmaceutical industry, contended for that limited utilization of compulsory licensing [37]. High-earnings countries feared that massive issuance of compulsory licensing would adversely change up the profits of the profession and would harm ale these businesses to analyze and develop within the lengthy term [36, 38]. Therefore, using compulsory licensing of pharmaceuticals was limited to highly infectious illnesses for example Aids/Helps with LMICs [39], and compulsory licensing aside from this restricted area was considered unjustifiable [38].
Compulsory licensing might be from a government in situations in which the patent holder is either not while using patent or perhaps is not utilizing it adequately, including pharmaceuticals sought after that aren’t fully available. When governments make an effort to issue compulsory licensing or non-governmental entities request a government to issue compulsory licensing, the end result is frequently home loan business prices, like the situation of the development of generics on the market [16, 31]. Although compulsory licensing isn’t a new idea, it’s received considerable debates as patent holders aim to advance their political stances over ip legal rights to gain access to to medicines. Particularly, opponents of compulsory licensing reason that compulsory licensing could be diametrically against the part from the patent system.
We designed this research to show the positive role from the matured ip system in attempting compulsory licensing. The issuance from the compulsory licensing of the patent needs a granted patent, the results of a functioning patent system. Therefore, a hyperlink exists between your compulsory licensing of patents and also the patent system. Within this study, we presented that using compulsory licensing is a valuable part from the patent system along with a legitimate tool for that government to make use of by demonstrating that compulsory licensing of pharmaceuticals is required particularly countries having a mature patent system.
To the understanding, this research is the first one to empirically analyse the connection between compulsory licensing and patent systems. We used a multivariate logistic model to regress the make an effort to issue compulsory licensing around the characteristics from the ip system (i.e., the PIPP index), controlling for macro context variables (i.e., region, population, earnings, and polity) in a country level. Interestingly, mature patent systems were positively connected with attempting compulsory licensing. How then might mature patent systems modify the issuance of compulsory licensing?
A brief history of compulsory licensing can help to explain these interesting results simply because they give a reason behind the presence of compulsory licensing inside a patent system. We’ll trace the origins from the compulsory licensing of patents in high-earnings countries and discuss interesting cases from Canada within the late 1980s.
Good reputation for compulsory licensing of patents
The next legislative cases within the 18-nineteenth century show high-earnings countries positively reviewed and introduced compulsory licensing to enhance the ip system and develop their very own industrial policy.
The compulsory licensing of patents was adopted through the compulsory licensing of copyrights [11]. In England, excessive cost as well as an inadequate supply by monopolies were acknowledged as a fiscal disease [32]. The Statute of Anne in 1710, referred to as world’s first copyright law, reflected this thought. Someone apart from who owns the copyright who determined that the book was over-priced could file a complaint against who owns the copyright to the court, along with a fine was enforced to who owns the copyright whether it violated the cost set through the court. Following a Statute of Anne, the U . s . States, particularly the condition of Connecticut, established the Copyright Act in 1783. The Copyright Act needed copyrighted books to become offered in a reasonable cost in sufficient quantities otherwise, it might be easy to file a complaint to the court, like the situation from the Statute of Anne. In addition, a legal court had the legal right to determine the amount and cost for that copyright owner when the copyright owner didn’t accept or rejected this type of request, a legal court had the legal right to possess the complainant print it. Therefore, the Copyright Act was evaluated like a legislative illustration of the world’s first system of compulsory licensing of copyrights [17].
The U . s . States, particularly the condition of Sc, established the Act for that Encouragement of Arts and Science, supplying rights and limitations enforced to authors (copyrights) in addition to inventors (patents) in 1784. The Act first devised compulsory licensing like a new remedy from the abuse from the patents when only invalidation from the patents existed. It ought to be noted the U . s . States have been more aggressive in working with patent abuse during the introduction of new industry. Similarly, England and Germany discussed compulsory licensing as measures to reduce the negative effects from the patent system and complement the shortcomings from the patent system in 1851 and 1853, correspondingly [18].
These trends favouring compulsory licensing in a variety of fields endured within the 1900s [5, 33]. The U . s . States invoked government use regularly to import pharmaceuticals within the 1960s. In addition, Canada utilized the compulsory licensing of pharmaceuticals not just for importation but in addition for local production to handle health expenditure [5, 34, 35]. For example, the Canadian government enacted compulsory licensing by amending the Patent Act to inspire local manufacturing from the pharmaceutical and market competition in 1923 [5]. In addition, there is public concern around the cost of pharmaceuticals which was considered as excessive through the 1960s. Because of the concern, the federal government made several amendments towards the Patent Act, which may have significant implications on patenting activities. Furthermore, the amendments permitted using compulsory licensing to fabricate or import pharmaceuticals which were limited to manufacturing before amendments [5]. The advantages of compulsory licensing are palpable in Canada, and numerous follow-on drugs have joined the marketplace.
However, the positive stance regarding compulsory licensing of high-earnings countries particularly altered within the last century, particularly throughout the negotiations for Journeys [36]. LMICs contended for the best to issue compulsory licensing for patented pharmaceuticals which were costly for his or her citizens, while high-earnings countries, usually affected by the pharmaceutical industry, contended for that limited utilization of compulsory licensing [37]. High-earnings countries feared that massive issuance of compulsory licensing would adversely change up the profits of the profession and would harm ale these businesses to analyze and develop within the lengthy term [36, 38]. Therefore, using compulsory licensing of pharmaceuticals was limited to highly infectious illnesses for example Aids/Helps with LMICs [39], and compulsory licensing aside from this restricted area was considered unjustifiable [38].
Meanwhile, WHO adopted the Doha Declaration in the 4th Ministerial Conference, and affirmed that compulsory licensing may be the right from the states from the WTO. The Doha declaration confirmed that compulsory licensing is meant being an efficient and simple instrument for LMICs to enhance use of needed medicines [34]. The Doha declaration was very efficient in issuance of compulsory licensing within the short term [1]. A couple of tries to issue compulsory licensing, including cases from low-earnings countries, happened following the declaration. However, the advantages of the declaration in issuing compulsory licensing wasn’t sustained within the lengthy term [1].
Role of compulsory licensing in patent systems and health systems
Our findings recommended a brand new role of compulsory licensing in current patent systems. Compulsory licensing is alternatively or complementally utilized in countries where mature patent systems were established. For example, China released “Measures for Compulsory Licensing of Patent Implementation” that integrated and updated ip laws and regulations to permit compulsory licensing this year [40]. Underneath the new measures, China’s Condition Ip Office (SIPO) may issue and terminate compulsory licensing for patents [41].
In addition, compulsory licensing within the patent system might be harmonized using the health system to secure use of essential medicines. In 2016, the very first time, the German Federal Patent Court particularly purchased a provisional compulsory license under Section 24 from the Patent Act. A Legal Court permitted permission seeker to carry on to promote an Aids drug which was made and offered since 2008 in Germany. Just before that, the patent holder had requested an initial injunction against permission seeker in 2015 according to an energetic patent granted this year, partly since the drug. The license seeker offered a voluntary license around the patent towards the patent holder, but unsuccessful. The license seeker then responded by requesting a compulsory license underneath the Patent Act [42]. Particularly, a legal court figured that the license seeker was titled to emergency relief and granted provisional compulsory licensing in line with the necessity of patients to achieve the drug available continuously throughout treatment [38].
Finally, compulsory licensing might be employed to export pharmaceuticals abroad based on paragraph 6 from the Doha declaration. However, cases around the compulsory licensing of pharmaceuticals for export are rare [33]. Meanwhile, an amendment towards the Journeys was enacted on The month of january 23, 2017. This amendment permitted countries to allow compulsory licensing to generic suppliers solely to fabricate and export medicines to countries missing production capacity [43]. It possesses a secure legal grounds for both potential importers and exporters to consider domestic legislation, and establishes the way to allow countries to import affordable follow-on drugs from countries where pharmaceuticals are patented.
Limitations from the study
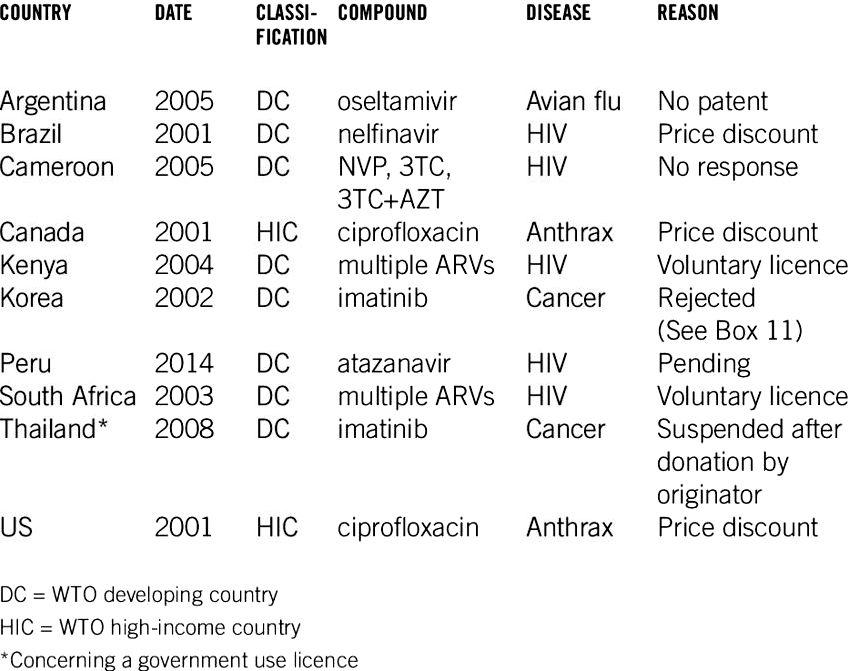
This research offers several limitations. First, this research used the existence of the attempted compulsory licensing of pharmaceuticals between 1995 and 2014. The amount of countries that attempted compulsory licensing throughout the study period was 24, comprising roughly 20% from the total of 139 countries analysed. Therefore, some might reason that compulsory licensing isn’t appropriate for quantitative research. However, it ought to be noted that empirical research might provide better implications on issuing compulsory licensing within the real life. These studies will lead to expanding the present knowledge of compulsory licensing. Second, due to scant available data and less cases on compulsory licensing, a mix-sectional analysis was put on investigate associations between attempting compulsory licensing and ip systems. However, panel analysis may well be a better approach to understand these associations. Third, this research used the database built by Boy and Lee (2018) to identify the existence of the attempted compulsory licensing of pharmaceuticals occurring between 1995 and 2014 [1]. Meanwhile, a far more comprehensive database regarding Journeys flexibilities, including compulsory licensing, was openly available [19]. We supplemented the database for that analysis. However, we excluded cases from Mongolia, Pakistan, Papua New Guinea, and also the Philippines which were according to non-openly available documents for example patent letters held by procurement agencies.
Resourse: https://globalizationandhealth.biomedcentral.com/articles/10.1186/

