Applying ip of pharmaceuticals in middle-earnings countries: a situation study of patent regulation in south america
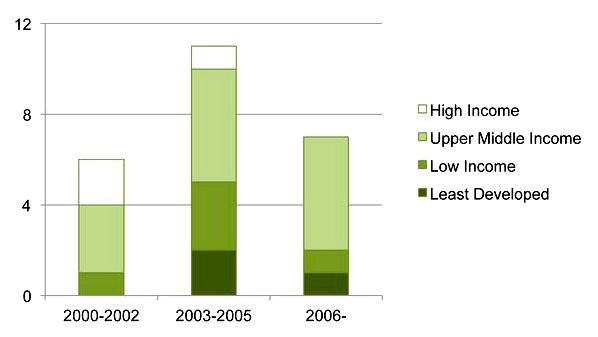
The security of pharmaceutical ip (IP) legal rights is among the most questionable debates in contemporary public health as countries need to balance incentives for drug development with involve supplying existence-saving drugs. Compliance with IP protections is required for people around the globe Trade Organization (WTO). However, due to the costs connected with IP implementation we ought to expect late and/or poor implementation in middle-earnings countries. Surprisingly, it was and not the situation in South america. The nation not just just fully implemented the WTO’s requirement but declined the elegance period granted for countries to evolve and incorporated extra IP protections, going against a coalition of local industrialists and activists. Notwithstanding, because the effects of IP rules unfolds, South america also promoted new alliances that tailored and adjusted the rules toward public health. We show arguments of foreign pressure and lobbying are exaggerated and call focus on domestic shifts, lengthy-term processes of regulatory decision, and political dynamics happening in the local level. By analyzing the situation of South america, we offer a nuanced contribution towards the discussion of IP implementation in middle-earnings countries and call focus on new types of government-society interactions in regulatory policy.
Resourse: https://read.dukeupress.edu/jhppl/article-abstract/41/3/423/13828/